Abstract
BACKGROUND:
Treatment options in patients with RRMM who are refractory to bortezomib (BORT) and lenalidomide (LEN) are limited. POM/LoDEX is approved in patients with RRMM after ≥2 prior treatment regimens (including both BORT and LEN), who relapsed on the last line of therapy. The aim of this ongoing, multi-center, non-interventional study is to prospectively collect real world data in RRMM patients treated with POM/LoDEX in an Austrian clinical routine setting.
PATIENTS & METHODS:
Adult patients with RRMM who had received ≥2 prior antimyeloma treatments (including LEN and BORT) who experienced disease progression on the previous line of therapy were enrolled and treated with POM/LoDEX until disease progression or unacceptable toxicity. Emphasis was put on the collection of safety data (adverse events [AEs]) and of efficacy data (objective response rate [ORR; ≥partial response, PR]; progression-free survival [PFS]). A source data verification level of 75% was reached in 74% of patients. Descriptive statistics were used to summarize the data.
RESULTS:
From 09/2014 to 06/2018, 63 patients were enrolled by 9 Austrian centers; 61 of them were eligible for analysis. At inclusion, the median age was 69 years (range 44-90); 15 patients (25%) were older than 75 years; 37 patients (61%) were male. Most patients (96%) had an ECOG performance status of ≤2. ISS stages were evenly distributed (I: 39%; II: 25%; III: 30%). The median number of prior lines of therapy was 4 (range 1-10). Prior treatments of almost all patients included an immunomodulatory drug (98%) and a proteasome inhibitor (97%). Half of the patients (49%) underwent a prior stem cell transplantation.
The most frequent starting dose of POM was 4 mg per day (82%); 43% of the patients received the full LoDEX dose of 40 mg weekly. The median number of POM treatment cycles was 10 (range 1-63). At cycle 10, 81% of patients still received POM at a dose of 4 mg, while only 19% still received LoDEX at the full dose of 40 mg.
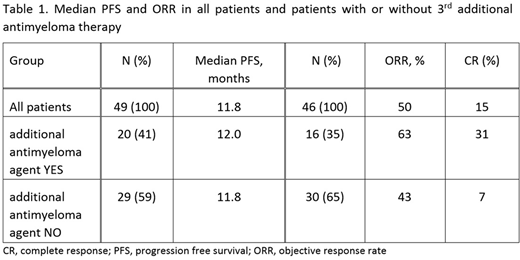
The median PFS for 49 evaluable patients was 11.8 months. Response information was available for 46 patients. The ORR with POM/LoDEX was 50%. Seven patients (15%) achieved a complete response (CR), 5 patients (11%) a very good PR, and 11 patients (24%) a PR; 4 patients still have an ongoing response.
A total of 23 patients (38%) received at least one additional antimyeloma agent at any time (57% started upfront; 43% delayed onset) during their treatment with POM/LoDEX. The ORR in this subgroup (n=16) was 63%. The median PFS and ORR stratified by application of a third or further antimyeloma agent are shown in Table 1.
Safety information was available for 47 patients (77%), 250 AEs were documented; of those, 94 (38%) were drug-related. Most AEs were non-serious (78%); 18% required hospitalization, 2% (not drug related) were fatal. The most common AEs were infections (22%) and neutropenia (9%). The most common reasons for treatment discontinuation were tumor progression (n=36, 59%), and death (n=4, 7%); one patient (2%) discontinued treatment due to toxicity. At the time of data cutoff, 13 patients (21%) were still on treatment.
CONCLUSION:
This interim analysis of real-world data demonstrates that POM/LoDEX is an effective and safe treatment in patients with RRMM.
Presented response data (PFS: 11.8 months; ORR: 50%) are somewhat better compared to data from earlier, randomized trials (MM-003, NCT01311687). Despite the limitations of a non-interventional study approach, these encouraging findings may also be a consequence of the increased practical experience clinicians have with POM/LoDEX today.
In contrast to LoDEX, dose reductions of POM are rare, underlining its good tolerability. As indicated by an increase of the ORR from 43 to 63% (subgroup analysis), efficacy of POM/LoDEX may be enhanced by addition of a third antimyeloma agent.
Lechner:Janssen: Consultancy, Honoraria; Novartis: Consultancy, Honoraria; Takeda: Consultancy, Honoraria; Celgene: Consultancy, Honoraria, Research Funding; Amgen: Consultancy, Honoraria, Research Funding; BMS: Consultancy, Honoraria. Greil:Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; MSD: Honoraria, Research Funding; BMS: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Sandoz: Honoraria, Research Funding; Merck: Honoraria, Research Funding; Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Astra Zeneca: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Honoraria, Other: TRAVEL, ACCOMMODATIONS, EXPENSES, Research Funding; Janssen: Other: TRAVEL, ACCOMMODATIONS, EXPENSES; Abbvie: Consultancy, Membership on an entity's Board of Directors or advisory committees. Hartmann:Novartis: Research Funding; Amgen: Research Funding; Roche: Research Funding; Celgene: Research Funding. Krauth:Amgen: Consultancy, Honoraria; Novartis: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; BMS: Consultancy, Honoraria; Celgene: Consultancy, Honoraria; Takeda: Consultancy, Honoraria. Gisslinger:AOP Orphan: Consultancy, Honoraria, Research Funding; Celgene: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; Novartis: Consultancy, Honoraria, Research Funding; Shire: Honoraria; Takeda: Consultancy, Honoraria. Vogl:BMS: Consultancy, Honoraria; Eisei: Consultancy, Honoraria; MSD: Consultancy, Honoraria; Janssen: Honoraria; Pfizer: Consultancy, Honoraria; Eli-Lilly: Consultancy, Honoraria; Celgene: Honoraria; Merck: Consultancy, Honoraria; Sanofi: Consultancy, Honoraria; Servier: Honoraria; Astellas: Honoraria; Bayer: Consultancy, Honoraria; Roche: Consultancy, Honoraria. Ohler:Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene: Membership on an entity's Board of Directors or advisory committees; Pfizer: Honoraria; Roche: Membership on an entity's Board of Directors or advisory committees, Research Funding. Greinix:Celgene: Consultancy; Amgen: Consultancy, Speakers Bureau; Therakos: Speakers Bureau; Roche: Speakers Bureau; Novartis: Consultancy, Speakers Bureau; Gilead: Speakers Bureau. Sormann:Celgene: Consultancy, Honoraria, Speakers Bureau; Amgen: Consultancy, Honoraria, Speakers Bureau; Takeda: Consultancy, Honoraria, Speakers Bureau. Andel:Roche: Research Funding, Speakers Bureau; Novartis: Honoraria; Amgen: Honoraria; Celgene: Honoraria. Rechberger:Celgene: Membership on an entity's Board of Directors or advisory committees; Roche: Membership on an entity's Board of Directors or advisory committees. Agis:Celgene: Consultancy, Honoraria; Amgen: Consultancy, Honoraria, Research Funding; BMS: Consultancy, Honoraria; Janssen: Consultancy, Honoraria, Research Funding; Prothena: Consultancy, Honoraria; Takeda: Consultancy, Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal